Glaxo Caught Hiding Paxil/Seroxat Data
 Drug manufacturer GlaxoSmithKline has been caught out submitting summary data to a British regulator, thus hiding details which show a much increased suicide risk associated with taking the SSRI drug Paxil(Seroxat).
Drug manufacturer GlaxoSmithKline has been caught out submitting summary data to a British regulator, thus hiding details which show a much increased suicide risk associated with taking the SSRI drug Paxil(Seroxat).The Medicines and Healthcare Regulatory Authority (MHRA) in the UK has banned SSRIs in children and adolescents, following an 18-month inquiry. However, it allowed use in adults, based in part on Paxil clinical trial data supplied by leading SSRI manufacturer GlaxoSmithKline.
But a newly-published study [abstract or pdf] reveals that the company did not submit the full detailed trial data to the regulator.
The Norwegian author of the report, Dr. Ivar Aursnes says GlaxoSmithKline provided a summary which added together suicide attempts and reports of patients feeling suicidal. That distorted the conclusions on the dangers of the drugs, says Dr Aursnes.
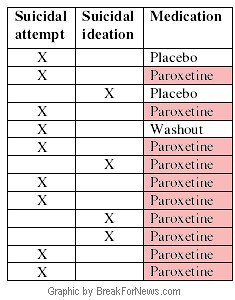
 He obtained the original trial results from the Norwegian regulatory authority and found that the data shows that in 16 trials there were seven attempted suicides among(916) people taking Seroxat, compared to one among those(512) given a placebo.
He obtained the original trial results from the Norwegian regulatory authority and found that the data shows that in 16 trials there were seven attempted suicides among(916) people taking Seroxat, compared to one among those(512) given a placebo.The MHRA brushed off Dr Aursnes' attempts to alert them to the deception, prompting him to conduct further research, resulting in the new study. It concludes that "the increased suicidal activity seen in children and adolescents on certain antidepressant drugs may well be present in adults".
The findings will be seized on by lawyers suing GlaxoSmithKline. The mental health charity Mind said the results were "extremely worrying" and confirmed what it had been arguing for years. "The drugs regulators may well have caused lives to be lost," said Sophie Corlett, policy director of the charity.
"Mind's own research has revealed that 50 per cent of the people who contacted us to report a reaction to Seroxat had experienced feelings of wanting to self-harm or commit suicide, and 58 per cent of these people said they had not experienced these feelings before they started taking Seroxat."
The MHRA should immediately issue a warning to doctors; should reopen their inquiry into SSRIs and should ask hard questions about Glaxo's suppression of the damaging drug trials data. But will they...?
Adapted from Guardian & Times UK

<< Home